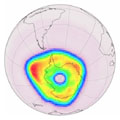
Ozone hole during 7 October 2008 as measured by Envisat |
The 2008 ozone hole – a thinning in the ozone layer over Antarctica – is larger both in size and ozone loss than 2007 but is not as large as 2006.
Ozone is a protective atmospheric layer found in about 25 kilometres altitude that acts as a sunlight filter shielding life on Earth from harmful ultraviolet rays, which can increase the risk of skin cancer and cataracts and harm marine life.
This year the area of the thinned ozone layer over the South Pole reached about 27 million square kilometres, compared to 25 million square kilometres in 2007 and a record ozone hole extension of 29 million square kilometres in 2006, which is about the size of the North American continent.
The depletion of ozone is caused by extreme cold temperatures at high altitude and the presence of ozone-destructing gases in the atmosphere such as chlorine and bromine, originating from man-made products like chlorofluorocarbons (CFCs), which were phased out under the 1987 Montreal Protocol but continue to linger in the atmosphere.
Ozone hole extension during the last 10 years |
As the polar spring arrives in September or October, the combination of returning sunlight and the presence of PSCs leads to a release of highly ozone-reactive chlorine radicals that break ozone down into individual oxygen molecules. A single molecule of chlorine has the potential to break down thousands of molecules of ozone.
Julian Meyer-Arnek of the German Aerospace Centre (DLR), which monitors the hole annually, explained the impact of regional meteorological conditions on the time and range of the ozone hole by comparing 2007 with 2008.
"In 2007 a less concentric and larger polar vortex led to an early onset of the ozone destruction in the sunlit parts of the polar vortex," Meyer-Arnek said. "Therefore, we saw an ozone hole formation in the beginning of September 2007 which corresponded to the average behaviour of the years 1995-2006."
"In 2008 a more concentric polar vortex led to a delay of the onset of the ozone destruction of about one week. The preconditioning of the polar chemistry was about the same for both years, although in 2008 the temperatures were slightly below the 2007 temperatures leading to slightly improved formation of PSCs," he continued.
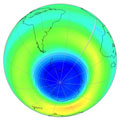
Chlorine activation early September 2008 |
Minimum values of the ozone layer of about 120 Dobson Units are observed this year compared to around 100 Dobson Units in 2006. A Dobson Unit is a unit of measurement that describes the thickness of the ozone layer in a column directly above the location of measurement.
DLR’s analysis is based upon the Scanning Imaging Absorption Spectrometer for Atmospheric Cartography (SCIAMACHY) atmospheric sensor onboard ESA’s Envisat, the Global Ozone Monitoring Experiment (GOME) aboard ESA’s ERS-2 and its follow-on instrument GOME-2 aboard EUMETSAT’s MetOp.
Scientists say that since the size and precise time of the ozone hole is dependent on the year-to-year variability in temperature and atmospheric dynamics, the detection of signs of ozone recovery is difficult.
"In order to detect these signs of recovery, a continuous monitoring of the global ozone layer and in particular of the Antarctic ozone hole is crucial," Meyer-Arnek said.
Average of total ozone values for September 2008 |
Studying the Envisat data, the students’ findings were in line with atmospheric scientists that the south polar vortex was more concentric in 2008 than in 2007, leading to a relatively late onset of ozone depletion, and that the size of this year’s hole is similar to previous years.
"This exercise led us to realise that although many questions have been answered and much has been learned about the stratospheric chemistry and atmospheric dynamics driving ozone hole behaviour, many new questions must be raised especially concerning ozone hole recovery," said Deborah C Stein Zweers, a post-doc satellite researcher from the Royal Netherlands Meteorological Institute (KNMI) who attended the course.
"We want to know when the ozone hole will recover, how its recovery will be complicated by an environment with increasing greenhouse gases and how atmospheric dynamics will shape future ozone holes. These and many other questions will attract the attention of our generation of scientists for the next several decades."Original here





No comments:
Post a Comment